
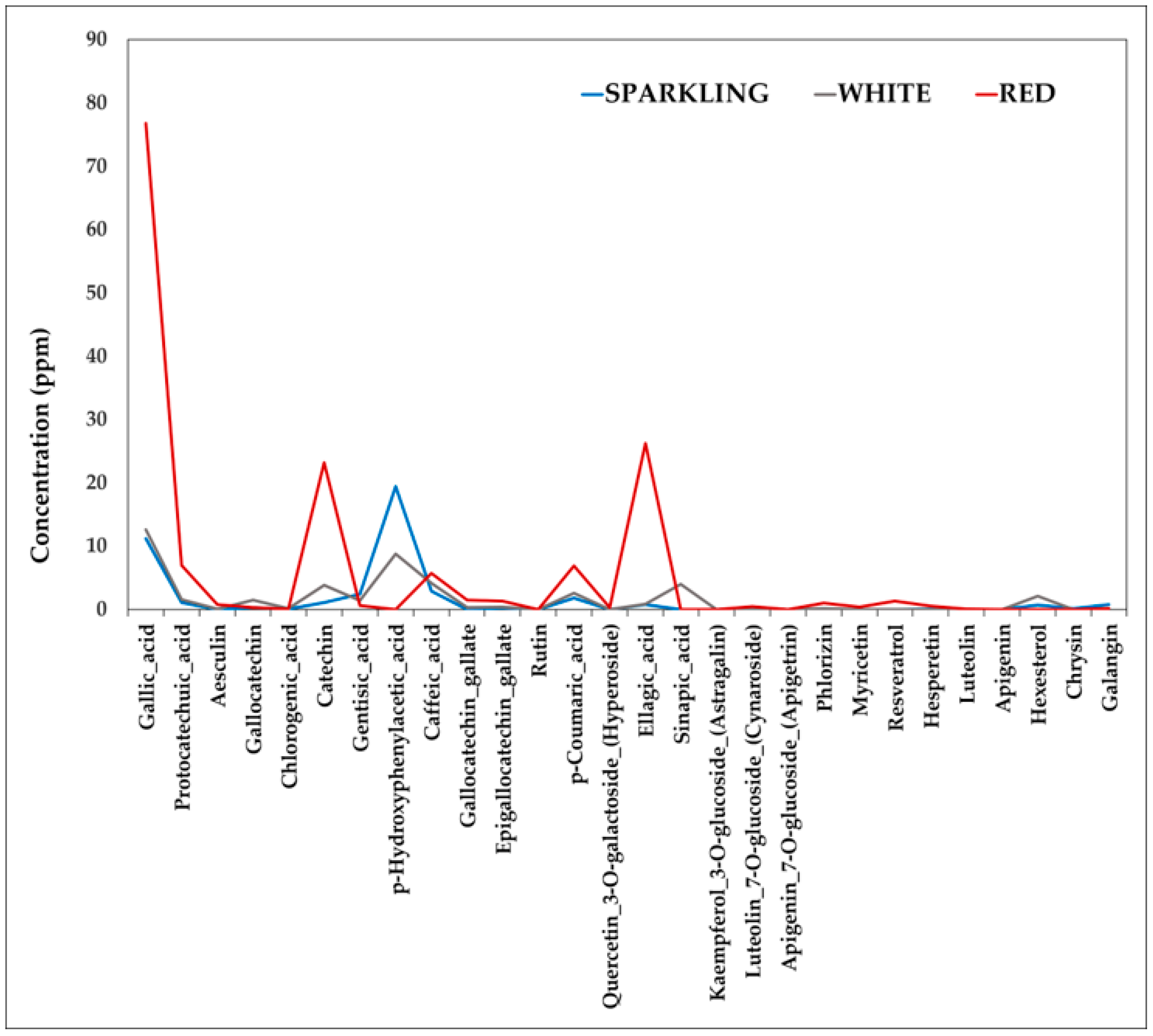

The mass percentage of a solution component is defined as the ratio of the component’s mass to the solution’s mass, expressed as a percentage: Percentages are also commonly used to express the composition of mixtures, including solutions. Mass PercentageĮarlier in this chapter, we introduced percent composition as a measure of the relative amount of a given element in a compound. In this section, we will introduce some other units of concentration that are commonly used in various applications, either for convenience or by convention. However, molarity is only one measure of concentration.
In the previous section, we introduced molarity, a very useful measurement unit for evaluating the concentration of solutions. Perform computations relating a solution’s concentration and its components’ volumes and/or masses using these units.Define the concentration units of mass percentage, volume percentage, mass-volume percentage, parts-per-million (ppm), and parts-per-billion (ppb).By the end of this section, you will be able to:


 0 kommentar(er)
0 kommentar(er)
